Downstream Purification Development
Process Assessment and Optimization
Drawing on extensive experience and understanding of process development, our development team has established a risk assessment system for antibody reducing to effectively mitigate risks during the CMC development process effectively.
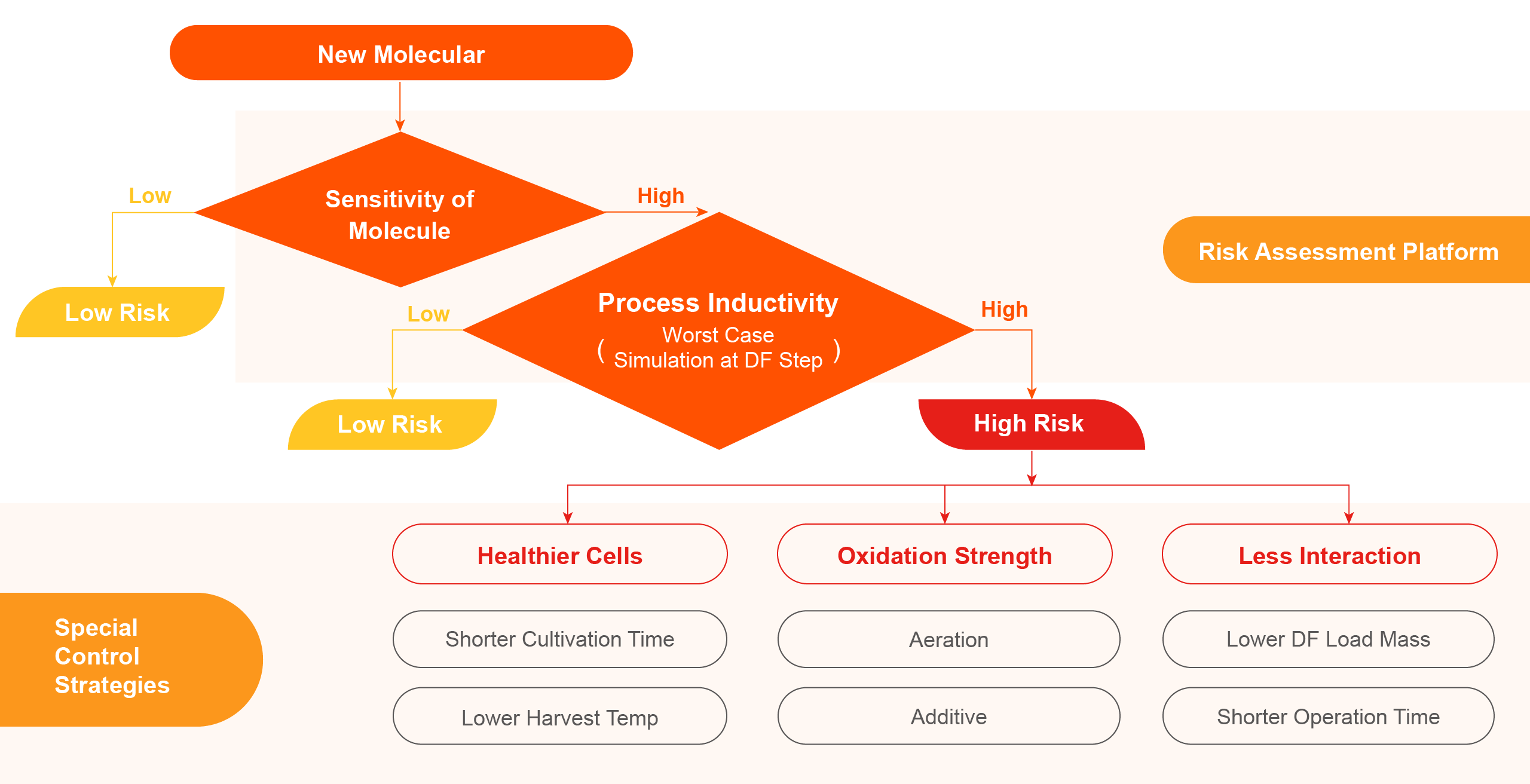
Process Characterization
Our development team employs process characterization methods that meet international submission requirements, including equivalence qualification of scaled-down models, application of various statistical methods in process characterization and data analysis, and definition/generation of process control strategies.
Case
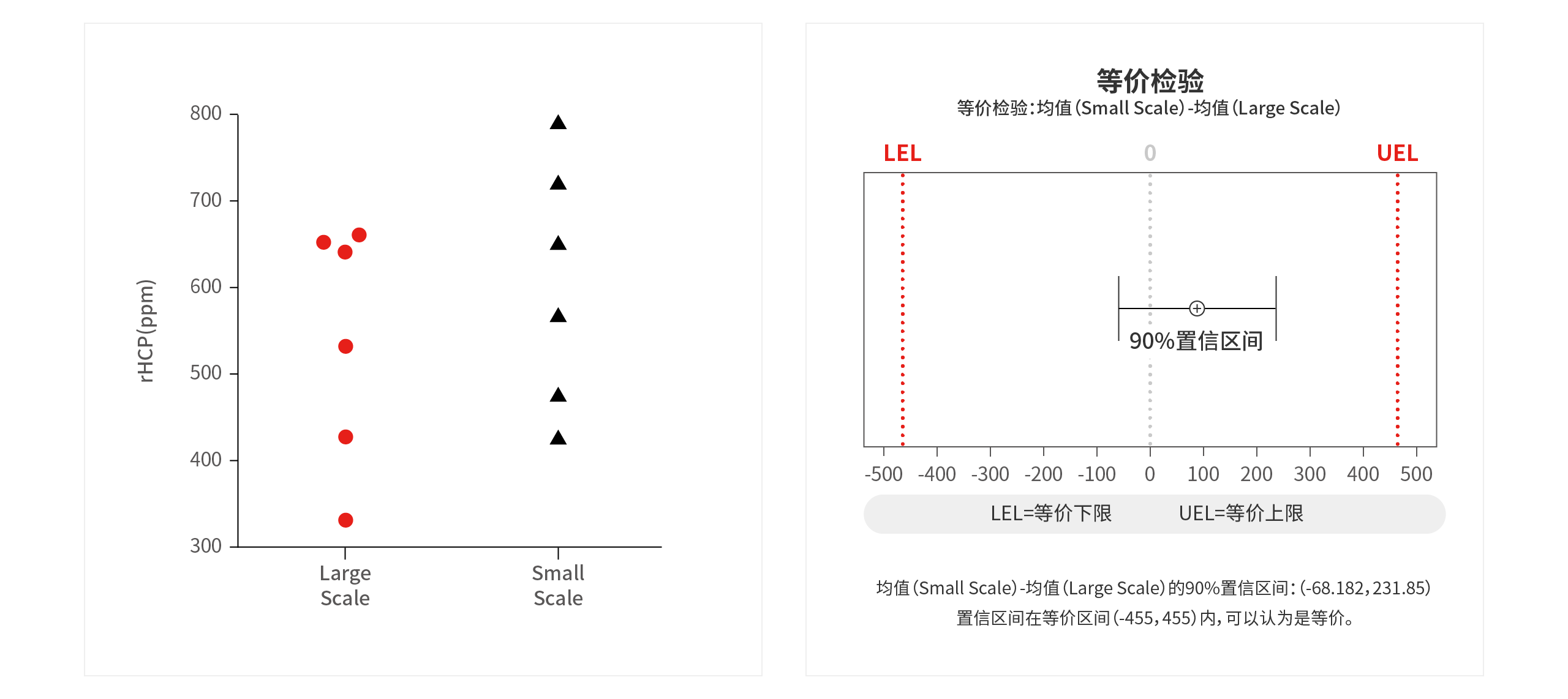
Project: Equivalence Assessment of Scale-Down Model for Affinity Chromatography (rHCP)
Localized Resin Screening Service
Adhering to the customer-first service philosophy, we not only shorten the time of process development through our well-established platform process, but we can also perform customized development of local resins based on cost requirements and preliminary testing data, aiming to achieve economic savings for our clients.

