ADC
Simplified & Integrated ADC Supply Chain
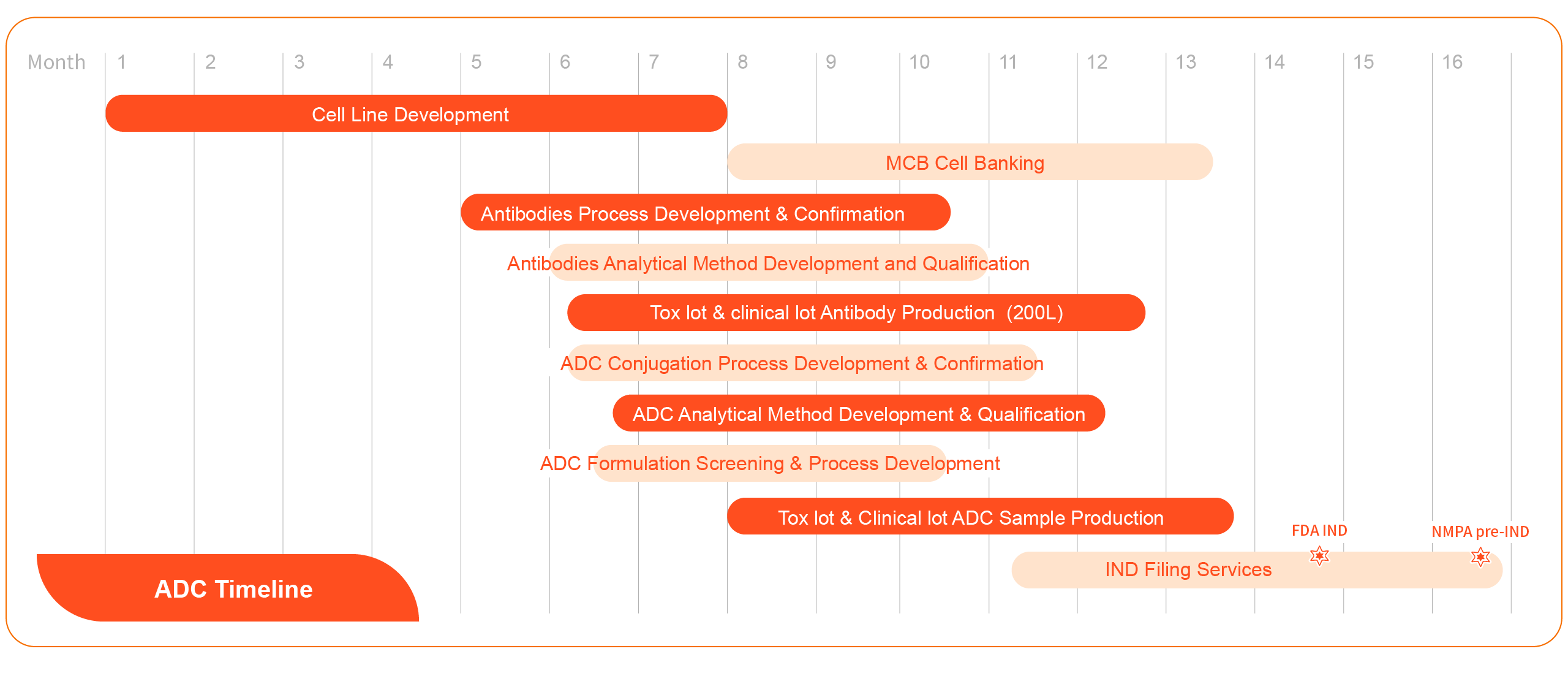
The dedicated manufacturing facility for the development and production of Antibody-Drug Conjugates (ADC) by Aton was officially put into operation at the end of 2023. The manufacturing lines comply with international GMP quality standards, providing comprehensive ADC drug CDMO services to biopharmaceutical companies worldwide. We aim to empower your ADC drug development and manufacture, becoming your most reliable partner.
Integrated CDMO service for ADC
20+ Antibodies
Ready To Go
(more Available with Sequence)
Bioconjugation & DP
Process Development
Analytical Method
Development & Qualification
DS & DP
Manufacturing
(OEB-5)
Complete ADC Production Capability
ADC Pilot Plant (DS+DP)
- Antibody Pre-treatment / Reduction Room
- Fill-finish and Lyophilization Room
- Bioconjugation & Purification Room
- Light Inspection Room
- Drug Weighting Room
- Cold Room ( 2-8°C, -40°C)
- Buffer Preparation Room
Pilot GMP Facility Equipped with OEB-5 Isolator for ADC
Bioconjugation capacity is up to
150L / Batch
• Compact line Composed of Washing
• Sterilizing & Drying, Filling, Sealing
• External Washing and Lyophilization
Pictures of Production Workshop
OEB5 Handling Suite
Payload Preparation Isolator
Comprehensive ADC Services from Development to Manufacturing
Development and Manufacturing Processes

Project Management of Full Product Lifecycle