Upstream Development
Process Optimization and Characterization
Comprehensive Optimization Strategies and Work Allocation
Based on our rich experience in biological development especially in the field of biosimilars, the upstream and downstream teams collaborate closely to optimize process yields and qualities. The optimization strategies and work arrangements are designed to balance yield, quality and cost from a general perspective. The optimizations include but are not limited to increasing product yields, modulating charge variants and monomer purities, and regulating glycosylation.
Rational Design of Process Characterization
Aton adheres to the concept of Quality by Design by persistently exploring more reasonable process characterization strategies. Risk analysis and assessment are integrated into whole product lifecycles. Scale-down models are continuously improved and advanced statistical analysis is used. Bioprocess is continuously monitored to ensure the effectiveness of process control strategies.
Case
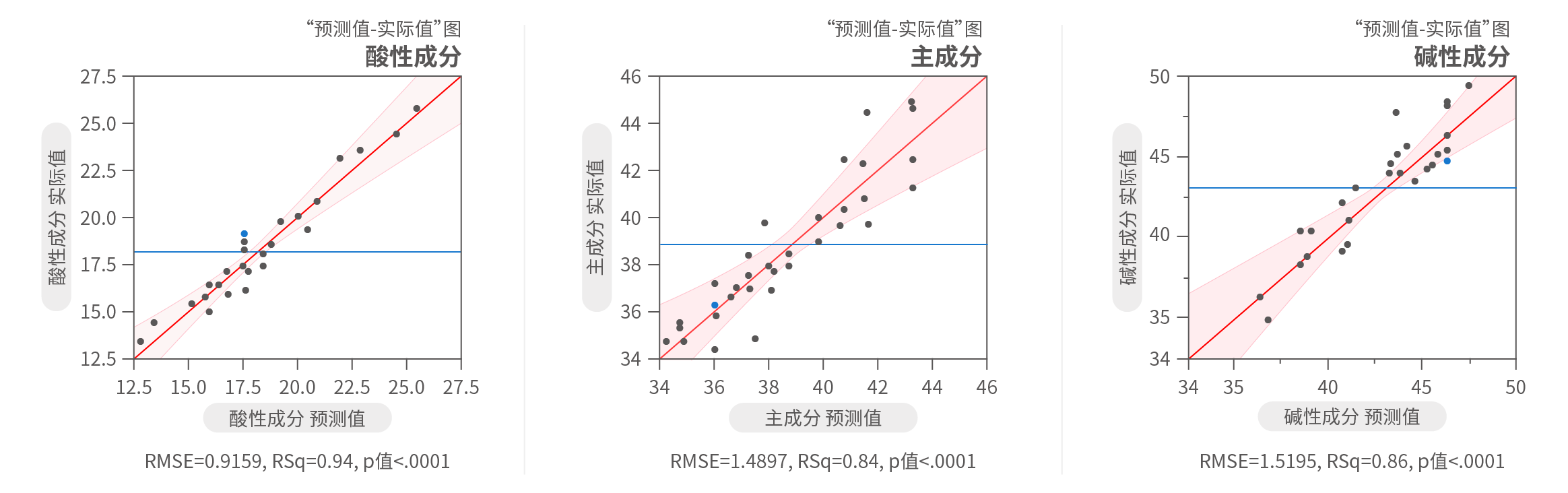
Case 1:Establishment of Scale-down Model
Content: Equivalence qualification of pCQAs and KPAs
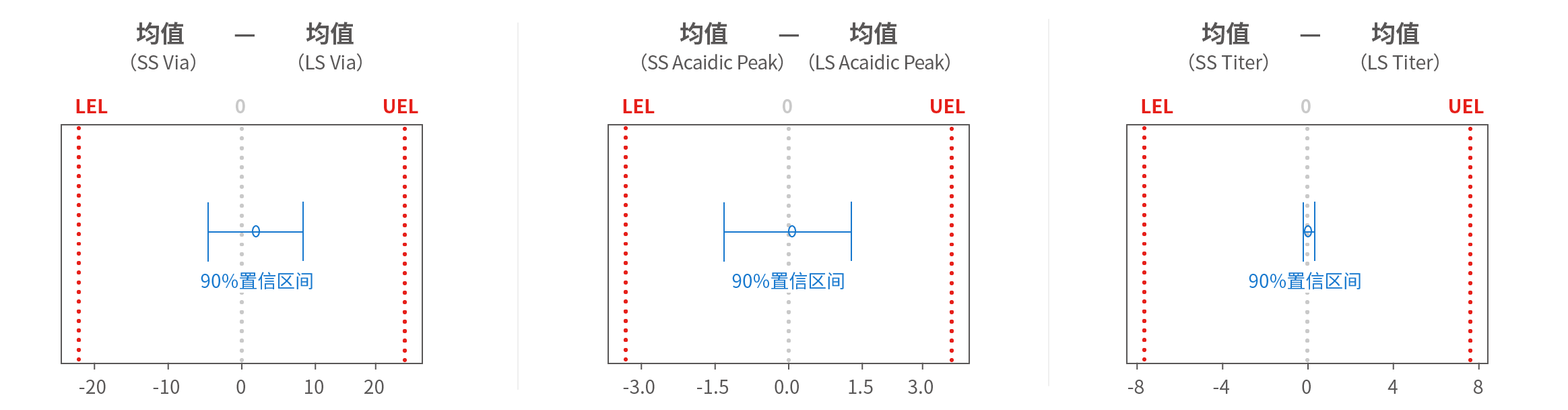
Case 2: Process Characterization Study
Content: Model fitting of Central Composite Design for Charge Heterogeneity Analysis
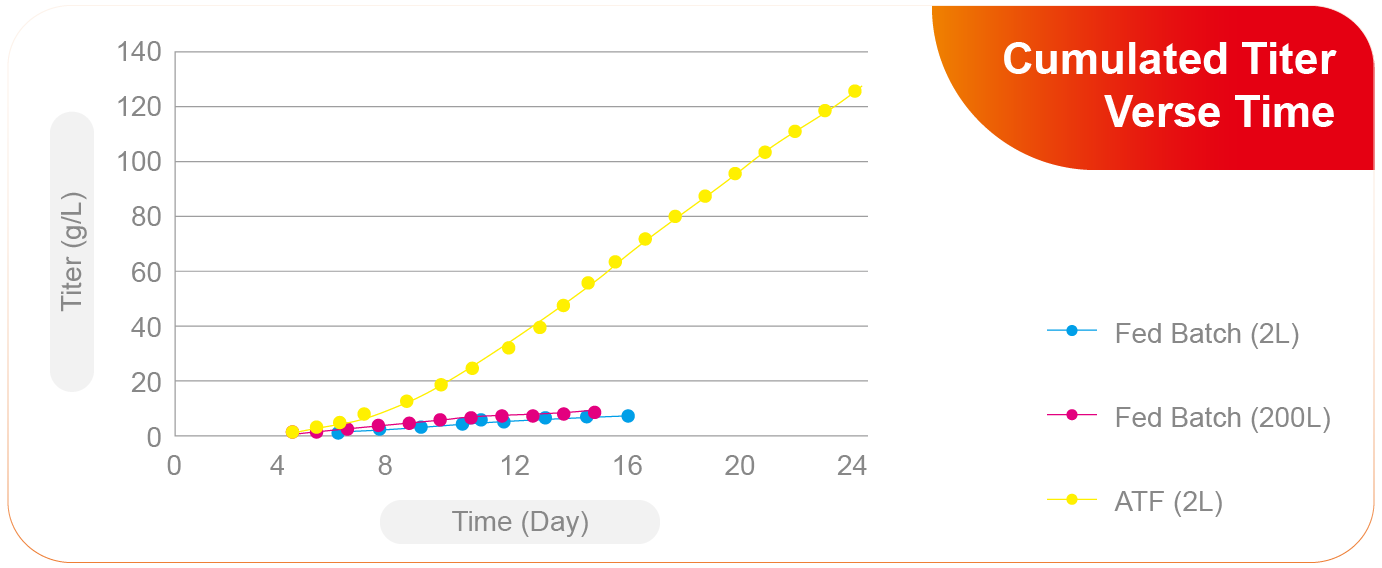
Compatibility with Traditional Fed-batch and New Generation Processes
Compatibility with Various Process Strategies
-
Not only traditional fed-batch process, Aton also has rich experience in high-density process intensification and perfusion process development.
Effective Enhancement of Process Yield
-
The new process effectively increased antibody yield by ten folds verse fed-batch process.
-
The new process is robust enough for manufacturing transfer.